

Also replace URL for the actual url of this page (The stay, ok?). How many electrons does single cesium atom lose All right Cesium is an Alkali Metal so it belongs to the first column of the periodic table. Now replace dd, mmmm and yyyy with the day, month, and year you browsed this page. The longest-lived radioisotopes are 135 Cs with a half-life of 2.
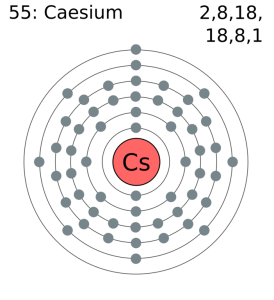
"Valence Electrons in Cesium (Cs) [& Facts, Color, Discovery. The atomic masses of these isotopes range from 112 to 151. To make your life (and citation) easier just copy & paste the information below into your assignment or essay: That gives credibility to your paper and it is sometimes required in higher education. CitationWhen you need to include a fact or piece of information in an assignment or essay you should also include where and how you found that piece of information. How about an incentive to share this post? (You will help other colleagues find this blog)ĭownload and enjoy this complete and colored periodic table for you to edit and enjoy. Need an editable periodic table to edit? Maybe add your school logo, work team or anything else to maker your paper look cool?Īlong with basic atom / element information (like Cesium valence electrons and all the other atomic data), it also comes with color coded info about: State (Gas, Liquid or Solid at room temperature), Groups/series details and much more. 1 Explain what happens to caesium atoms and to oxygen atoms when caesium reacts. How a small number of atoms can be joined and form completely different substances.Video Are you having trouble understanding the basics of atomic elements? This video will walk you through: Want to learn more details and data about Cesium (Cs)? Check my Elements Comprehensive List. it loses its outermost electron quite easily, but the 2nd ionization enthalpy is very high. Note: Caesium has the least ionization enthalpy value among IA group elements, i.e. SourcesFound in pollucite and as trace in lepidolite.ĭiscoveryDiscovered By: Gustov Kirchoff, Robert Bunsen How many valence electrons does cesium lose Therefore the number of valence electrons of caesium is 1. Name OriginLatin: coesius (sky blue) for the blue lines of its spectrum. Since it ionizes readily, it is used as an ion rocket motor propellant. Also used in producing photoelectric devices and atomic clocks. UsesUsed as a 'getter' to remove air traces in vacuum and cathode-ray tubes. In the case of Cesium the valence electrons is 1. Now let's check the facts about Cesium.ĭescriptionVery soft, light gray, ductile metal. Ok but how many valence electrons does an atom of Cesium have? A valence electron is an outer shell electron and may participate in the formation of a chemical bond.


 0 kommentar(er)
0 kommentar(er)
